cmv pcr blood test bottle|cmv diagnosis protocol : manufacturing Changes and New Tests Explore the most recent updates to our Laboratory Test Directory in one convenient location. Review important information about upcoming or current Hotlines, CPT code changes, new tests, and immediate activations. Test Resources Find general guidance on ARUP specimen preparation and handling, specimen transportation, test turnaround times, critical . Resultado da Watch Aline Mineiro tube sex video for free on xHamster, with the hottest collection of Latina HD hardcore porn movie scenes to download and stream!
{plog:ftitle_list}
WEB爱词霸权威在线词典,为您提供time的中文意思,time的用法讲解,time的读音,time的同义词,time的反义词,time的例句等英语服务。
Cytomegalovirus (CMV), Qualitative, PCR. TEST: 138693. CPT: 87496. Print Share Include LOINC® in print.
Dried Blood Spot: Whole blood collected on newborn screening card (3/16 inch punch). Transport punch in an ARUP standard transport tube. Tissue: Transfer to a sterile container .CMV tests check for signs of the virus in the blood, sputum, or other body fluids. CMV testing can help those at risk for complications get the treatment they need. While there is no cure for .Changes and New Tests Explore the most recent updates to our Laboratory Test Directory in one convenient location. Review important information about upcoming or current Hotlines, CPT code changes, new tests, and immediate activations. Test Resources Find general guidance on ARUP specimen preparation and handling, specimen transportation, test turnaround times, critical .Tube Type Tests Instructions Blood Culture Bottles Blood Culture Ensure aseptic technique. Collect Aerobic, then . CMV PCR PPT Manganese Trace Metals CMV Serology Serum SST Measles Serology Serum SST CMV Viral Load PPT Microarray EDTA-3mL Coagulation profile Sodium Citrate Mumps Serology Serum SST .
This test is even helpful in detecting CMV infection in newborns. Contact Private Blood Tests London for same day cmv pcr test in London. Blood London have been providing CMV PCR Blood Test UK on a self-referral basis to patients in Central and Greater London for over 20 years. Simply walk-in weekdays between 9am and 6pm or on weekends between .
what is a cmv test
labcorp cmv test
The enzyme-linked immunosorbent assay (ELISA) is the most common serologic test for measuring antibody to CMV. Tests for congenital CMV in newborns. Polymerase chain reaction (PCR) on saliva is the standard laboratory test for diagnosing congenital CMV infection. Urine is usually collected and tested for confirmation.Test Directory; CMV PCR; CMV PCR . In the immunocompromised blood is the preferred sample, although other samples may be appropriate (e.g. CSF in suspected CMV encephalitis). . (EBV) PCRs. CMV PCR is not normally useful in the diagnosis of primary CMV infection in the immunocompetent, where serology with CMV IgM testing is indicated. For .The performance of the US9 CMV real-time PCR (laboratory-developed test) is summarized in the Table: Table. Performance of the US9 CMV real-time PCR assay following testing of prospective clinical samples (n=200) US9 CMV PCR. Consensus result. Positive. Negative. Kappa. Sensitivity. Specificity. Positive. 45. 0. 0.99. 97.8 (87.6-99.9)Quantitative CMV DNA PCR can be used for early detection of CMV reactivation, primary infections, and monitoring response to treatment. About Cytomegalovirus Clinically significant Cytomegalovirus infection frequently develops in immunocompromised patient populations (e.g. hematopoietic stem cell transplantation, solid organ transplant and HIV).
The use of plasma instead of whole blood in these tests requires rapid separation to keep the DNA from degrading with resultant fragmentation . A recent study has shown that once plasma is separated from whole blood, . (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype Amplicor CMV Monitor test in transplant .CMV is also the most common cause of congenital viral infection in humans. Quantitative PCR methods may be useful in monitoring CMV replication in immunosuppressed patients or in determining the viral load of CMV in amniotic fluid. This is a quantitative molecular test, with a linear range of 200-2,000,000 IU/mL. INTRODUCTION. Cytomegalovirus (CMV) is a common infection, and, although serious disease is rare in immunocompetent individuals, CMV is a major pathogen for immunocompromised patients, including solid organ transplant recipients, hematopoietic cell transplant recipients, human immunodeficiency virus (HIV)-infected patients, and patients .

The CMV test is a low-risk procedure that involves a simple blood draw. You don’t need to take any special steps to prepare for it. Your doctor can use it to learn if you have an active CMV .The standard laboratory test for diagnosing congenital CMV infection is polymerase chain reaction (PCR) on saliva, with urine usually collected and tested for confirmation. The reason for the confirmatory test on urine is because most CMV seropositive mothers shed CMV virus in .
cmv test results explained
Blood: Centrifugation upon arrival, remove plasma/serum from cells. Plasma/serum specimens may be stored and/or transported: 2 to 8°C for up to 6 days-20°C ± 2°C for up to 12 weeks; Sterile Body Fluid: If CSF has cellular material, centrifuge & aliquot clear CSF.Freeze at -10°C or colder and ship on dry ice. During pregnancy and after delivery. If you're pregnant, testing to determine whether you've ever been infected with CMV can be important. Pregnant women who have already developed CMV antibodies have a very small chance of a reactivation infecting their unborn children.. If your doctor detects a new CMV infection while you're pregnant, a prenatal .
This assay is not intended to be used as a screening test for CMV or as a diagnostic test to confirm the presence of CMV infection. Development Philosophy The Abbott RealTi m e CMV assay uses PCR technology .Concentration of the CMV DNA in a patient's plasma sample is determined by a ratio of the intensity of the fluorescent dye from the cleaved CMV target sequence probes and that from the DNA-QS target probe detected throughout the PCR process.(Package insert: cobas CMV-Quantitative nucleic acid test for use on the cobas 6800/8800 Systems.Cytomegalovirus (CMV) is a common infection among both children and adults. In the United States, nearly one in three children is infected by 5 years of age, and more than 50% of adults become infected by 40 years of age. CMV can be . So people that are immunocompromised can also benefit from CMV negative blood. How Rare is CMV Negative Blood. Regular testing is conducted on blood donations to check for CMV antibodies. Because CMV exposure is so prevalent, CMV negative blood is very special. If up to 85% of adults in the US have been exposed by age 40, that means only 15% of .
foxconn c-3598
Some patients with CMV end-organ disease may not have detectable levels of CMV DNA in peripheral blood. In such cases, CMV detection via qualitative PCR in clinical samples taken from the organ system in question (e.g. urine, CSF or respiratory tract sample) or immunohistochemical staining of tissue biopsy specimens may prove useful.Babies born with congenital CMV may have tests to check their kidneys, liver, brain, eyes and hearing, and regular follow-up appointments until they're around 5 years old. . You can pass it on through contact with body fluids, including saliva, blood, breast milk, pee and poo, and through sex. CMV can only be passed on when it's active. The . Another test, called the CMV PCR test, looks for the presence of CMV DNA in the body. Prenatal Testing. . There are different types of CMV tests, including blood tests and testing of bodily fluids such as saliva, urine, or tissue samples. The most common method used to test newborns is a rapid CMV test that involves collecting a small sample .The cobas ® CMV is an in vitro nucleic acid amplification test for the quantitative measurement of cytomegalovirus (CMV) DNA in human EDTA plasma. The cobas ® CMV is intended for use as an aid in the diagnosis and management of CMV in solid organ transplant patients and in hematopoietic stem cell transplant patients. The test can be used in these populations to .
Positive qualitative PCR tests should be followed up with a quantitative CMV PCR test, which is not available at PHO’s laboratory. If available, testing these patients with a quantitative PCR test as the primary testing approach is preferred. . Do not freeze whole blood; store and ship refrigerated within 48h of sample collection. Plasma .Sample type: Urine Test name: CMV PCR (In house) a.k.a. CMV PCR Condition / Indication: Diagnosis of congenital CMV infection. Special precautions & notes: Obtain current blood sample from mother if congenital infection is suspected. Two independent urines taken as soon as possible after birth but no later than 3 weeks of life should be taken to investigate congenital . Negative test results do not preclude CMV infection or tissue-invasive CMV disease, and test results should therefore not be the sole basis for patient management decisions. . Plasma samples separated from whole blood within 24 hours of collection may be stored and/or transported for up to 6 days at 2-8°C or up to 12 weeks at -20°C±2°C .
CMV tests may be ordered, along with tests for influenza, mononucleosis (mono), and EBV (Epstein-Barr virus), when a pregnant woman or an immune-compromised person has flu- or mono-like signs and symptoms, such as:. Fatigue, weakness; Sore throat; Swollen lymph nodes; Fever; Headache; Muscle aches; CMV tests may be ordered at regular intervals after .Blood: Centrifugation upon arrival, remove plasma/serum from cells. Plasma/serum specimens may be stored and/or transported: 2 to 8°C for up to 6 days-20°C ± 2°C for up to 12 weeks; Sterile Body Fluid: If CSF has cellular material, centrifuge & aliquot clear CSF.Freeze at -10°C or colder and ship on dry ice.
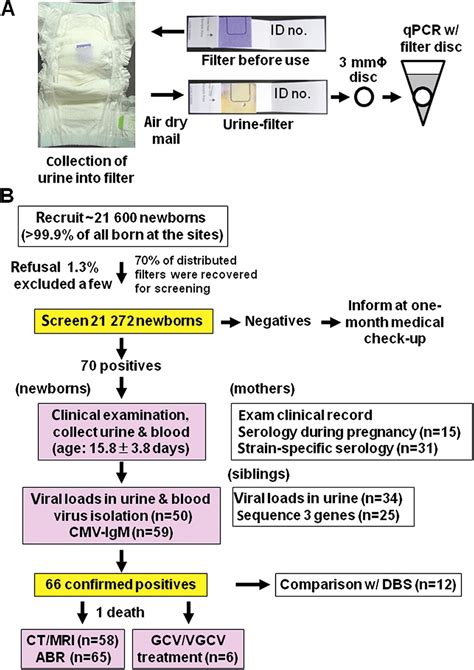
cmv pcr time
webXVIDEOS ANANZINHA COM 1.20!!! DE PURA SAFADEZA SEDUZIU E ACABOU ENTRANDO NA PICA DE UM COROA CASADO free
cmv pcr blood test bottle|cmv diagnosis protocol